Carbon Bonds Overview & List | How Many Bonds Does Carbon Make? - Video & Lesson Transcript | Study.com

Carbon Bonds Overview & List | How Many Bonds Does Carbon Make? - Video & Lesson Transcript | Study.com
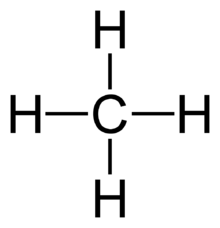
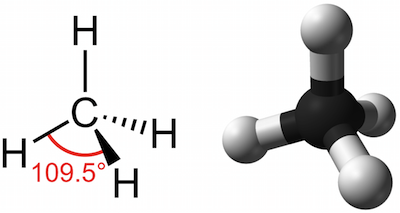
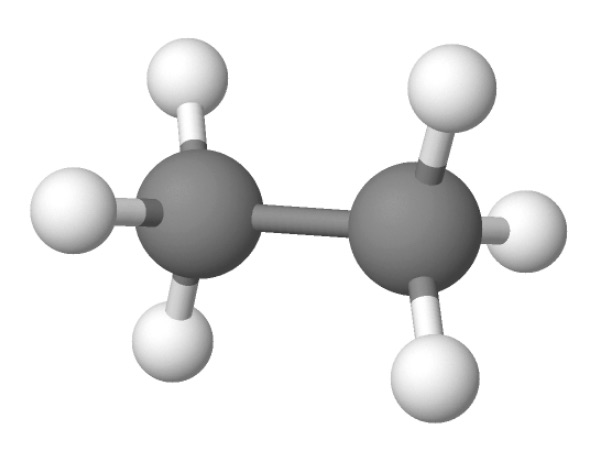
The four single bonds of a carbon atom in CH_4 are directed toward the corners of what shape? | Socratic

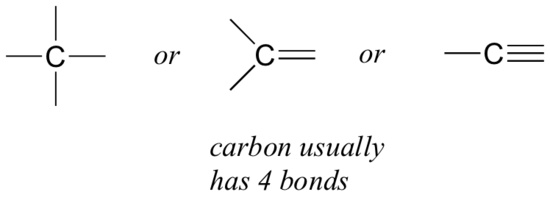
organic chemistry - Why are triple covalent carbon to carbon bonds drawn linearly in skeletal structure? - Chemistry Stack Exchange
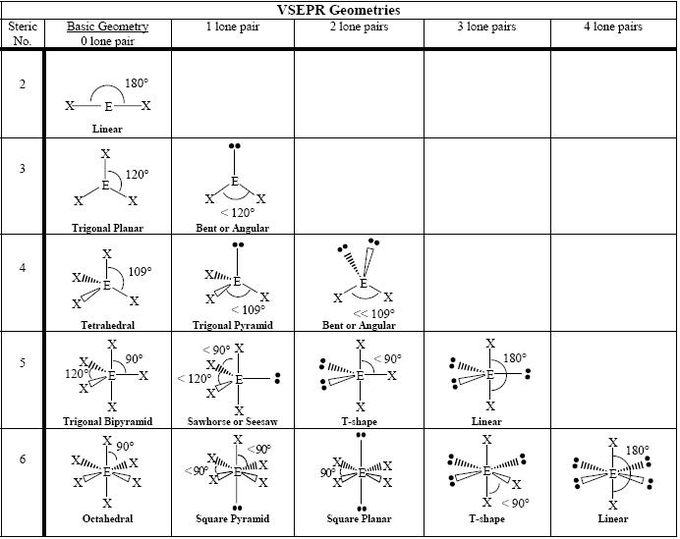
The four single bonds of a carbon atom in CH_4 are directed toward the corners of what shape? | Socratic
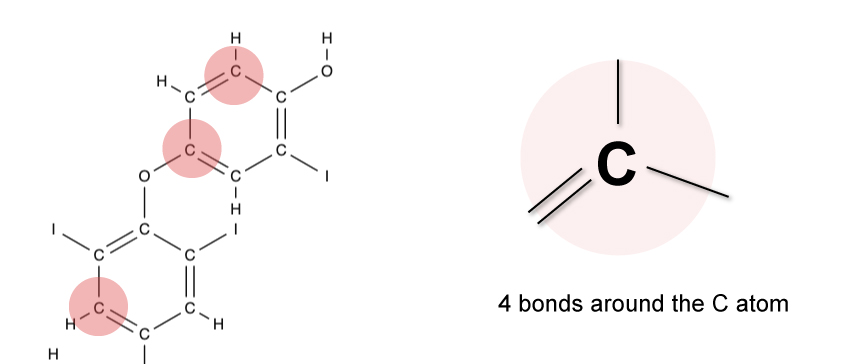
Carbon to Carbon - Single, Double & Triple Bonds - Surfguppy - Chemistry made easy for visual learners
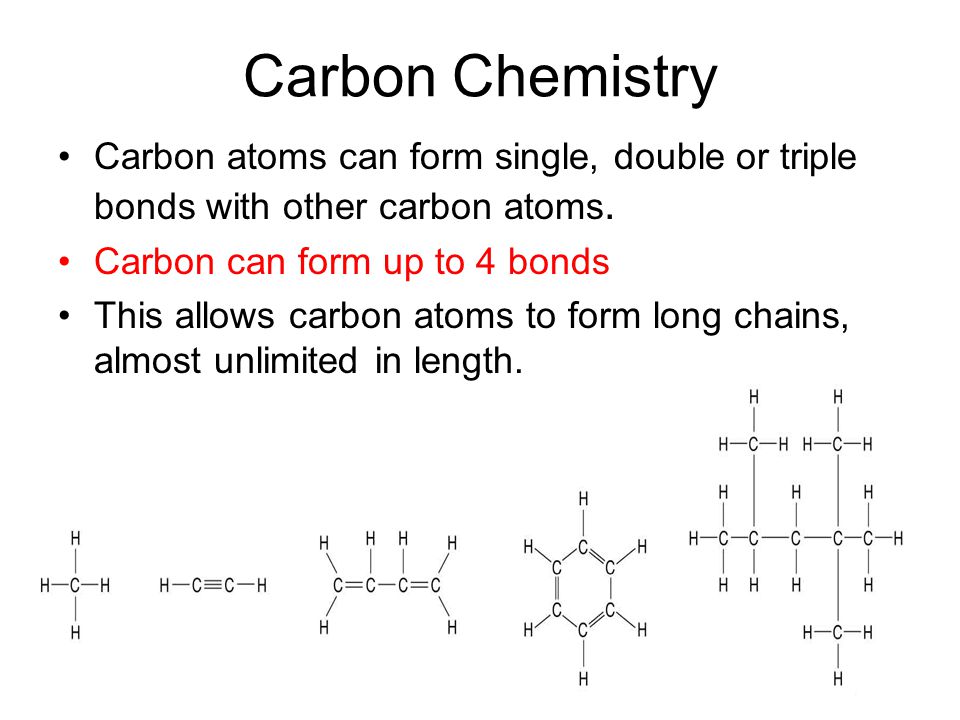
Carbon Chemistry Carbon atoms can form single, double or triple bonds with other carbon atoms. Carbon can form up to 4 bonds This allows carbon atoms to. - ppt video online download

Section 20.1 Saturated Hydrocarbons 1.To understand the types of bonds formed by the carbon atom 2.To learn about the alkanes 3.To learn about some common. - ppt download

Compliance constants for the carbon-carbon bonds in cyclobutane and... | Download Scientific Diagram