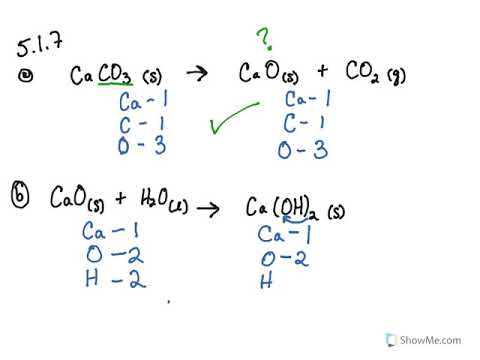
Hydroxide + carbon dioxide is equal to calcium carbonate + water balance the equation numerically - Science - - 12540547 | Meritnation.com
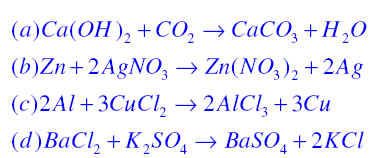
Write the balanced chemical equations for the following reactions. (a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water | Pushpender86's Blog

Passing Carbon Dioxide Gas Through Calcium Hydroxide Solution Activity Diagram Drawing Easily - YouTube
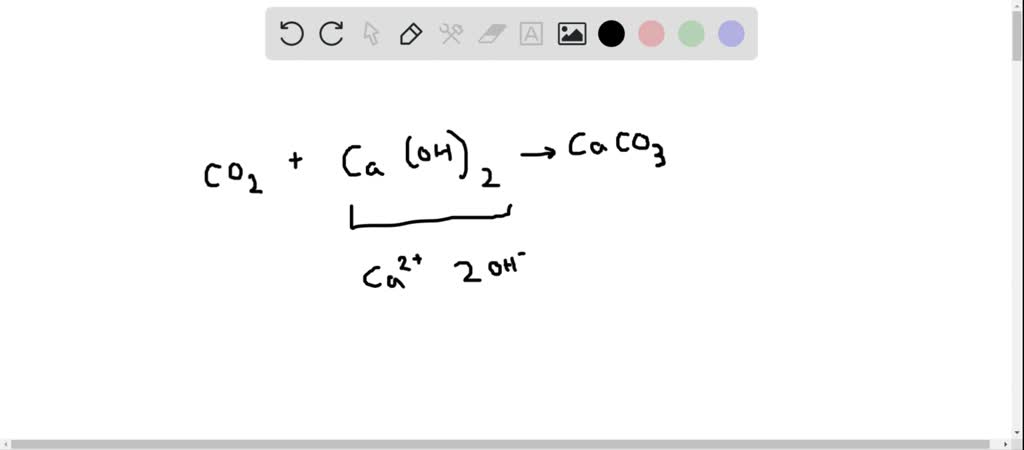
SOLVED:When carbon dioxide is bubbled through a clear calcium hydroxide solution, the solution appears milky. Write an equation for the reaction and explain how this reaction illustrates that CO2 is an acidic

Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.

Energy-efficient chemical regeneration of AMP using calcium hydroxide for operating carbon dioxide capture process - ScienceDirect

Carbon dioxide test. Test tube of limewater with carbon dioxide gas being bubbled through. The limewater, a solution of calcium hydroxide in water, is Stock Photo - Alamy

EP0610781A1 - Carbon dioxide absorber and its manufacturing method using concrete sludge - Google Patents

science chemistry precipitation reaction carbon dioxide | Fundamental Photographs - The Art of Science

Reactions of carbon dioxide - Gas chemistry - (CCEA) - GCSE Chemistry (Single Science) Revision - CCEA - BBC Bitesize

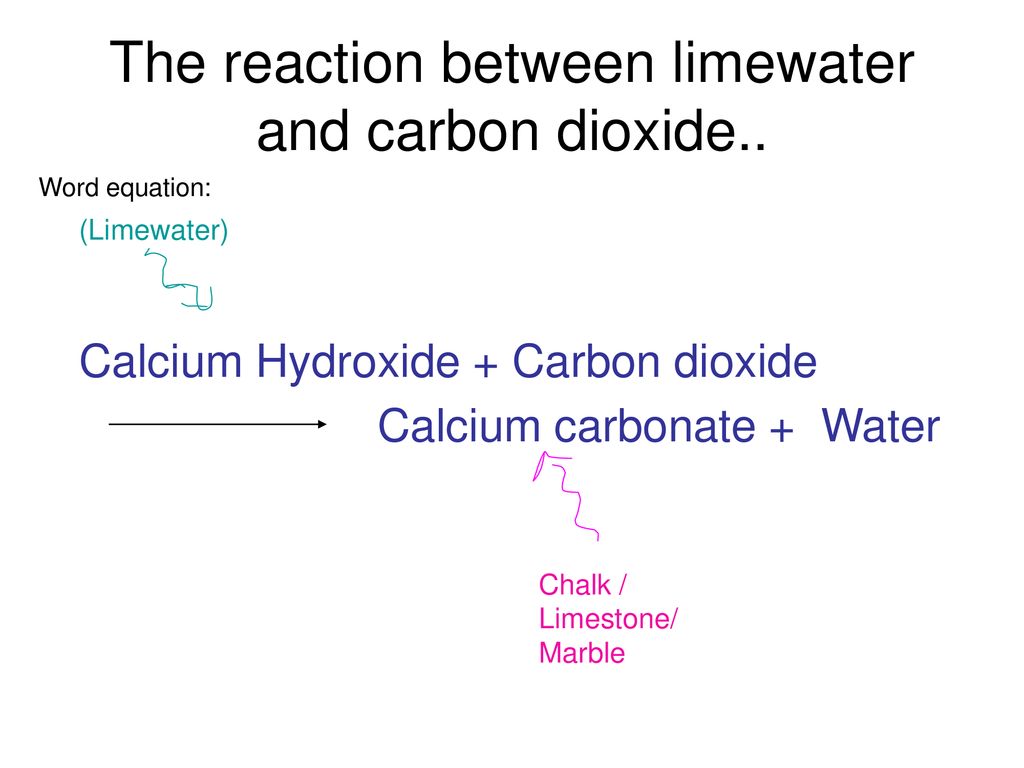
AQA GCSE Science & Additional Science Chemistry 1 Topic 2 Hodder Education Revision Lessons Limestone and building materials Click to continue. - ppt download