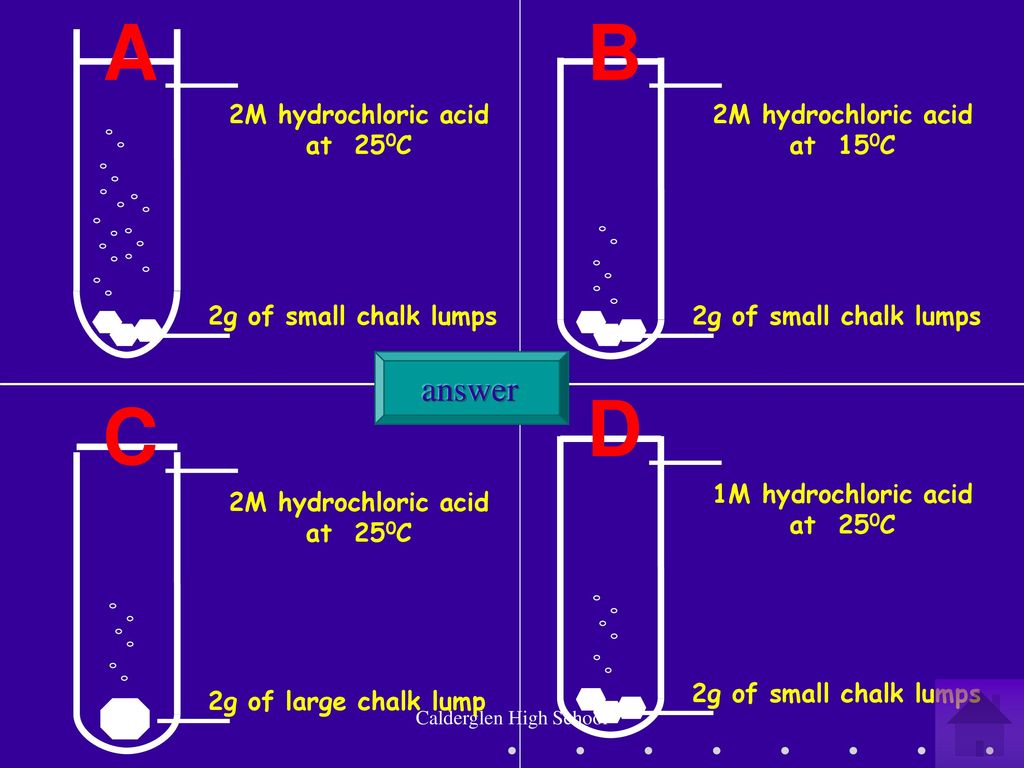
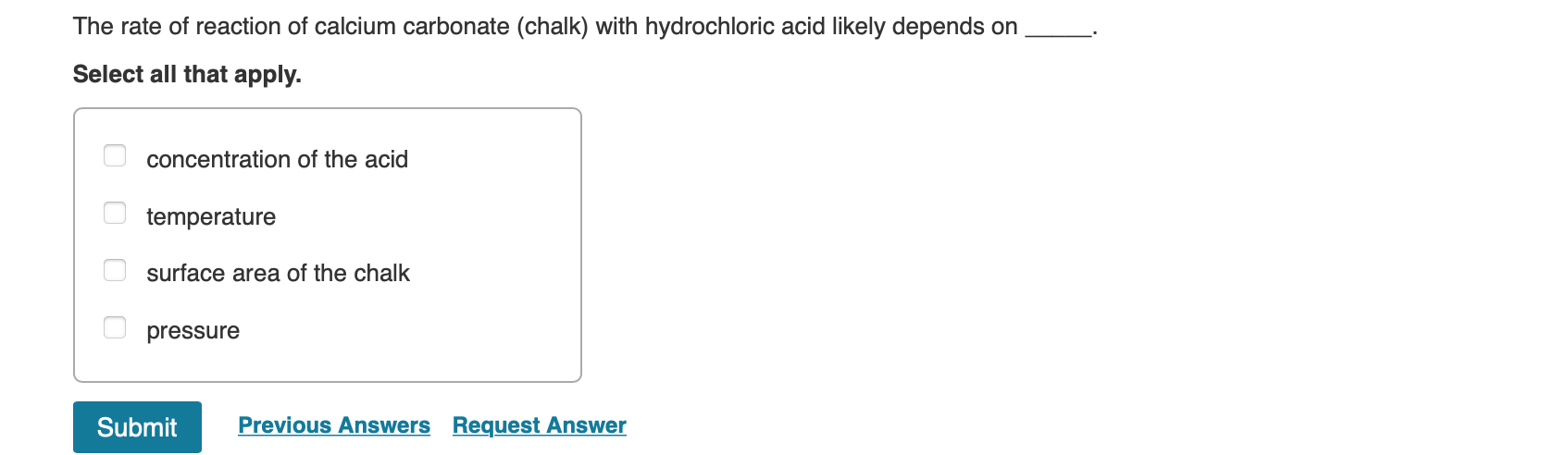
Write a balanced chemical equation the reaction of calcium carbonate and dil. hydrochloric acid.{ CaCO }_{ 3 }+2HClrightarrow { CaCl }_{ 2 }+{ CO }_{ 2 }+{ H }_{ 2 }O{ CaCO }_{

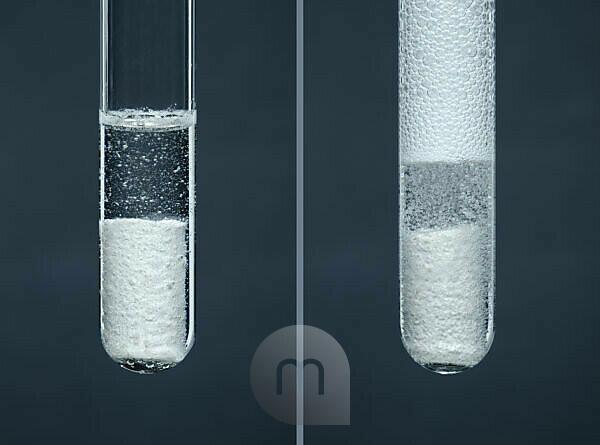
Reaction of chalk and acid. This is an example of an acid-carbonate reaction. Chalk contains calcium carbonate, and the acid here is hydrochloric acid Stock Photo - Alamy

SOLVED: When calcium carbonate reacts with hydrochloric acid, a surprising reaction occurs: CaCO3(s) + 2HCl(aq) -> H2O(l) + CaCl2(aq) (a) Balance the chemical equation above. (b) If a 2.82-g stick of chalk